March 23, 2012 - Abbott is warning consumers and healthcare professionals about two separate instances of counterfeit Vicodin ES product purchased via the Internet.
Vicodin ES is a pain relief medication sold in the United States through secure drug supply channels to reach consumers. Abbott works to preserve the ability to fill and refill legitimate prescriptions, issued through legitimate physician-patient relationships, using on-line technology. Abbott also works to prevent the sale of Vicodin class products through rogue Internet sites and is issuing this alert to help consumers and healthcare professionals identify counterfeit Vicodin.
The counterfeit product drug and package do not match that of Abbott's FDA- approved Vicodin ES (hydrocodone bitartrate and acetaminophen). The counterfeit tablets are off-white with the markings F and P embossed on one side of the tablet. Removal of the fake Vicodin ES label revealed tablet markings and packaging which are believed to be Fenak Plus. Fenak Plus contains a different active ingredient (diclofenac) than Vicodin ES and is used to treat pain and fever. (Source: Abbott Labs http://www.abbott.com/vicodin-consumer-alert.htm)
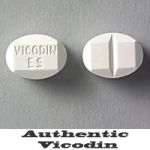
Vicodin ES Tablet: Distinct tablet shape with Vicodin ES markings embossed on one side (not actual size)
Authentic Abbott Vicodin ES Blister Package Label: Abbott Vicodin ES is packaged in a 25 tablet blister package. Each individual blister has detailed information and a bar code.
FDA approved blister packs of Vicodin ES [100-count (4 X 25 tablet blisters)] are packaged together and intended for hospital use.
Identifying the Counterfeit Label: Vicodin ES is not available in a 10 tablet blister. Multiple misspellings and missing information. Incorrect "Rx Only" designation and the Abbott logo is missing.
This label for Fenak Plus was found underneath the counterfeit Vicodin ES label shown above.
 |