May 29,2102 -- FDA warns of counterfeit Adderall.
The U.S. Food and Drug Administration is warning consumers and health care professionals about a counterfeit version of Teva Pharmaceutical Industries Adderall 30 milligram tablets that is being purchased on the Internet.
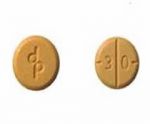
Authentic Adderall 30 mg tablets produced by Teva are round, orange/peach, and scored tablets with "dp" embossed on one side and "30" on the other side of the tablet. Teva's Adderall 30 mg tablets are packaged only in a 100-count bottle with the National Drug Code (NDC) 0555-0768-02 listed.
Adderall, which is approved to treat attention deficit hyperactivity disorders (ADHD) and narcolepsy, is a prescription drug classified as a controlled substance -- a class of drugs for which special controls are required for dispensing by pharmacists.
There are misspellings on the counterfeit Adderall package label as follows: "NDS" instead of "NDC", "Aspartrte" instead of "Aspartate", and "Singel" instead of "Single".
Authentic Adderall 30 mg tablets produced by Teva are round, orange/peach, and scored tablets with "dp" embossed on one side and "30" on the other side of the tablet.
The counterfeit Adderall tablets are round, white and do not have any type of markings, such as letters or numbers. Any product that resembles the tablets or the packaging in the photos below and claims to be Teva's Adderall 30 mg tablets should b
The Adderall 30 mg product may be counterfeit if the product comes in a blister package.
There are misspellings on the counterfeit Adderall package label as follows: "NDS" instead of "NDC", "Aspartrte" instead of "Aspartate", and "Singel" instead of "Single".
 |