Apr 28, 2012 -- Bevacizumab (trade name Avastin, Genentech/Roche) is an angiogenesis inhibitor, a drug that slows the growth of new blood vessels. It is licensed to treat various cancers, including colorectal, lung, breast (outside the USA), glioblastoma (USA only), kidney and ovarian.
The Food and Drug Administration (FDA) is investigating counterfeit reports, but hasn't received any reports from patients complaining of side effects that appear linked to counterfeit Avastin.
The Wall Street Journal reported that two batches of counterfeit Avastin have been found in the US. One contained salt and starch.
A 400-milligram vial of Avastin - the size that was counterfeited, costs $2,400, according to a Genentech spokeswoman.
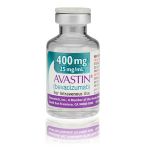
Avastin boxes sold in the US are labeled in English, and are labeled as made by Genentech with a six-digit lot number with no letters.
The expiry date is formatted as a 3-letter month and 4-digit year, e.g. JUL 2014;
The counterfeit boxes have writing in French, and lot numbers, on the boxes or vials, read B86017, B6011 or B6010.
The lot number on the carton and vial should be 6 digits with no letters.
The expiry date is formatted as a 3-letter month and 4-digit year, e.g. JUL 2014.
The date of manufacture is not printed on the carton or vial.;
The counterfeit Avastin does not look the same as the Avastin approved for sale in the US.
The counterfeit boxes have writing in French, and lot numbers, on the boxes or vials, read B86017, B6011 or B6010.
The counterfeit boxes have writing in French, and lot numbers, on the boxes or vials, read B86017, B6011 or B6010.
 |